CRO | CDMO
NGS | PCR | LAMP | Microfluidic chip


The best choice in CDMO for IVD Reagents and Expertise.
CRO | CDMO
CRO | CDMO
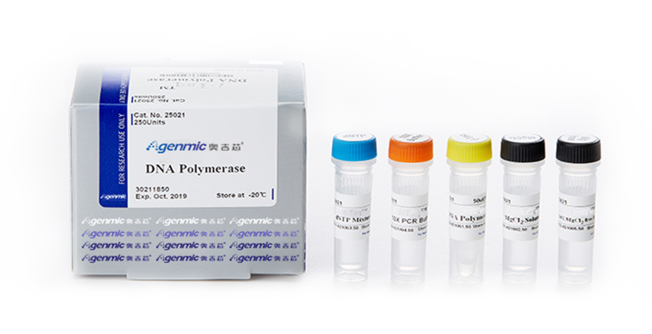
Agenmic is best known for its research and development of IVD reagents on a wide range of diagnostic platforms including NGS, PCR, LAMP, and Microfluidic chip. Our reputation is built on, not only bringing the reagents to the market, but also accumulating the validation data by the amounts of clinical evidence over many years.
Professional in CRO & CDMO.
Customization and Co-Development

QMS Production

Regulatory Services

Independent Lab for Clinical Trial

Go2Market
We Support your Team at any Product Stage
We Support your Team at any Product Stage
R & D
PRODUCT DEVELOPMENT
Hundreds of IVD products
Multiple platforms
One –Stop solution
Testing
MARKET COMPLIANCE
Self-operated independent labs
Large number of clinical samples
Diverse pre-clinical tests
Professional regulatory team support
Production
cGMP MANUFACTURING
International standard compliance
Flexible production volume
Quality supply chain and on-time delivery
Whole workflow QMS
Go2Market
INCREASING CUSTOMERS AND
BUSINESS DEVELOPMENT
Nationwide distribution channels
Market compliance
Product installation and training
After-sales support
Post-market surveillance (PMS)
OUR WORKFLOW
OUR WORKFLOW
Full Lifecycle Management by Agenmic
01

Concept & Feasibility

Main Concept Idea
Technical Research + Review
Market Analysis + Risk Analysis
Development Plan
First Design Proposal
QMS
02

Prototyping

Proto Development
Reviews And Alternatives
Safety lssues
Product Validation And Evaluation
QMS
03

R&D

Pilot Production
Regulatory Validation
Clinical Trial
Documentation + Protocols
QMS
04

Enhancement

End-To-End Validation
Regulatory Roadmaps
Documentation
QMS
05
Market Compliance & Go2Market
Regulatory Submission
CE NMPA and other Regulatory Marking
OMS Validation
PMS Guidelines

CDMO Capabilities
CDMO Capabilities
Technology Platforms
NGS, PCR, LAMP, Microfluid chip
Manufacture
10,000 sqm standard GMP workshop
ISO 13485, ISO 9001, NMPA QMS
Production license of Class II/III IVD reagents
Independent Labs
7 independent laboratories in China
Large number of clinical samples for testing
Professional R&D team and rich
co-development experience
Application Fields
Human microbiome, Noninvasive Prenatal Testing
(NIPT), Cancer Research and Immuno-oncology,
Animal study, Food science, Agrigenomics,
Environment, etc.

Agenmic Factory Profile
Agenmic Factory Profile
● Agenmic has three factories located in Fuzhou, Nantong, and Suzhou.
● ISO 13485 and GMP certified.
● With a total 10,000 ㎡ facility, and 5 Class 100,000 cleanrooms.
● More than 10 IVD production lines with the annual capacity of 20 million tests.
● Multiple production platforms: NGS, PCR, LAMP, Microfluid chip, etc.
Total factory area: 10,000㎡
Warehouse area: 1,000㎡
QC laboratory: 800㎡
R&D laboratory: 500㎡
LDTs Measurement Laboratory
Our Co-development Products
Our Co-development Products
>100
Reagents
>300
Components
We manufacture a wide range of assays and assay components for biopharma and diagnostics companies around the world.